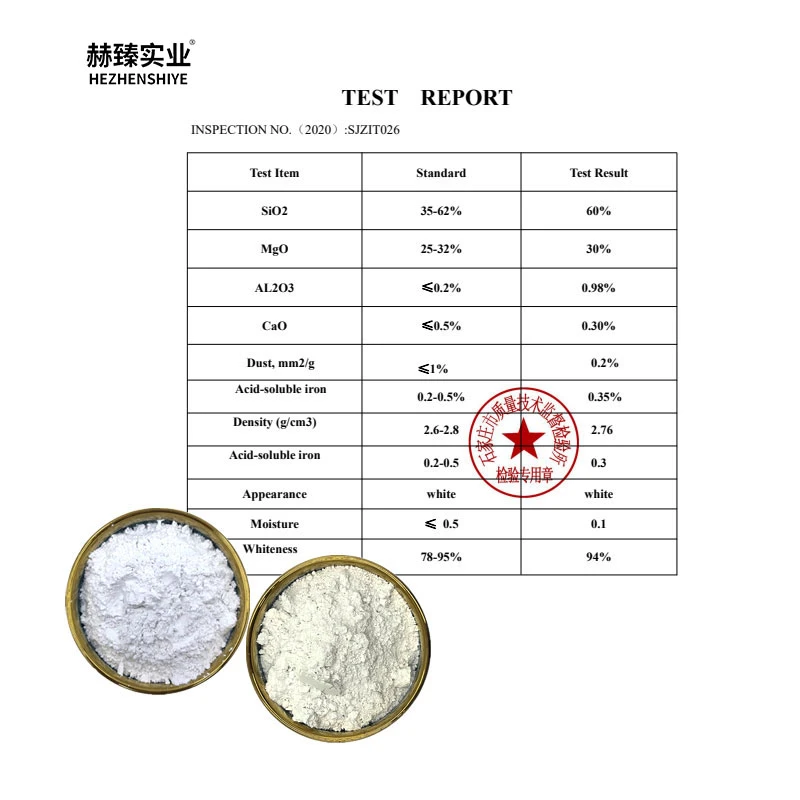
- Introduction to silicon dioxide applications
- Technical advantages in pharmaceutical formulations
- Leading manufacturer comparison
- Custom formulation solutions
- Real-world application case studies
- Regulatory and safety profile
- Tablet performance optimization insights

(silicon dioxide what is it used for)
Silicon Dioxide: What Is It Used For in Tablet Manufacturing?
Silicon dioxide (SiO₂), a naturally occurring compound, serves as a critical functional excipient across pharmaceutical manufacturing. Commercially available as colloidal silicon dioxide, this nanoscale material typically ranges between 7-40 nm in particle size. In tablet production, it functions primarily as:
- A flow enhancer that reduces interparticulate friction
- An anti-caking agent preventing moisture absorption
- A stabilizer for sensitive active ingredients
- A compression aid improving tablet hardness
With global pharmaceutical excipient markets projected to reach $11.5 billion by 2028 (Grand View Research), colloidal silicon dioxide represents approximately 18% of glidant usage. Regulatory approvals include FDA GRAS status, USP/NF compliance, and inclusion in the European Pharmacopoeia monograph.
Technical Advantages in Pharmaceutical Processing
The unique properties of colloidal silicon dioxide deliver measurable production improvements:
Bulk density improvements averaging 15-25% allow for smaller tablet sizes while maintaining dosage integrity. Comparative studies show 60% reduction in tablet ejection forces versus alternative glidants, extending tooling lifespan. Stability testing demonstrates:
- Moisture protection up to 65% RH without caking
- Oxidation reduction for sensitive APIs like statins
- Dissolution profile maintenance over 36-month stability
Process efficiency gains include approximately 15% faster tablet press speeds and a 2-5% reduction in rejected tablets due to picking/sticking defects.
Leading Colloidal Silicon Dioxide Manufacturers
| Manufacturer | Product Name | Particle Size | Bulk Density (g/cm³) | Moisture Adsorption |
|---|---|---|---|---|
| Cabot Corporation | CAB-O-SIL® M-5P | 0.2-0.3 μm | 0.03-0.04 | 60% at RH 75% |
| Evonik Industries | AEROSIL® 200 | 12 nm | 0.04-0.06 | 65% at RH 75% |
| Grace Materials | Syloid® 244FP | 2.7 μm | 0.24-0.29 | 58% at RH 75% |
| PQ Corporation | LUDOX® AM | 22 nm | 0.05-0.07 | 62% at RH 75% |
Process efficiency gains include approximately 15% faster tablet press speeds and a 2-5% reduction in rejected tablets due to picking/sticking defects.
Formulation-Specific Solutions
Colloidal silicon dioxide concentration ranges from 0.1-5% w/w depending on formulation challenges. Effective applications include:
- 0.1-0.5%: Standard tablet flow improvement
- 0.5-1.5%: High-API load formulations (>80%)
- 2-3%: Hygroscopic compound stabilization
- 3-5%: Brittle fracture reduction in chewables
Advanced formulation techniques show dry granulation processes benefit from late-stage addition to prevent shear-induced densification. Direct compression systems achieve optimal results when silicon dioxide blends with APIs before adding binders.
Industry Application Case Studies
Practical implementations demonstrate colloidal silicon dioxide used in tablet formulation solving production issues:
Multivitamin Producer Challenge: Capping during compression at 50,000 tablets/hour
- Added 0.8% colloidal silicon dioxide to premix
- Reduced tablet ejection force by 42%
- Achieved production speed increase to 70,000 tablets/hour
Hormone Therapy Tablet: Moisture degradation during storage
- Incorporated 1.2% surface-treated silica
- Maintained
- Reduced packaging costs by eliminating desiccant requirements
Regulatory and Safety Profile
Colloidal silicon dioxide meets stringent global standards for pharmaceutical use. Safety considerations include:
- EP 9.0 monograph acceptance (Ph Eur 04/2018:0873)
- FDA Inactive Ingredient Database approval for oral dosage forms
- GRAS classification up to 2% in food applications
Occupational exposure limits remain at 6 mg/m³ for respirable particles (OSHA). Studies indicate no significant systemic absorption with oral administration when particle size exceeds 100nm.
Maximizing Tablet Performance: Why Colloidal Silicon Dioxide Is Used
The functionality of colloidal silicon dioxide used in tablets addresses multiple manufacturing challenges simultaneously. Recent formulation studies demonstrate 5-year tablet production consistency when colloidal silicon dioxide is incorporated at optimal concentrations. The versatility extends to emerging areas:
- Continuous manufacturing processes requiring constant flow properties
- Orally disintegrating tablets needing rapid wetting without binders
- High-potency APIs where content uniformity is critical
Pharmaceutical engineers increasingly specify surface-modified grades that provide both lubrication and API dispersion, potentially reducing excipient counts in formulations. For tablet performance optimization, silica remains indispensable.
(silicon dioxide what is it used for)
FAQS on silicon dioxide what is it used for
围绕核心关键词「Silicon Dioxide」的5组英文FAQs问答:Q: What is silicon dioxide commonly used for?
A: Silicon dioxide (SiO₂) is primarily used as an anti-caking agent in powdered foods and supplements to prevent clumping. It also serves as a stabilizer in pharmaceuticals and a thickener in cosmetics. Additionally, it reinforces materials like glass, ceramics, and concrete in industrial applications.
Q: Why is colloidal silicon dioxide used in tablets?
A: Colloidal silicon dioxide acts as a glidant and flow agent in tablet manufacturing, ensuring powders blend smoothly during compression. It improves tablet uniformity by reducing friction between particles. Its small particle size also aids in rapid dissolution for faster drug delivery.
Q: What role does colloidal silicon dioxide play in tablet formulation?
A: In tablet formulation, colloidal silicon dioxide enhances powder flowability and prevents ingredient segregation during mixing. It stabilizes active compounds and extends shelf life by adsorbing moisture. This results in consistent tablet hardness and weight control.
Q: Is silicon dioxide safe in consumer products?
A: Yes, silicon dioxide is generally recognized as safe (GRAS) by the FDA and approved globally for use in food, supplements, and medicines. As an inert substance, it passes through the body undigested when used within regulated limits. Safety concerns mainly arise from inhalation risks in occupational settings (e.g., mining).
Q: How does colloidal silicon dioxide differ from regular silicon dioxide?
A: Colloidal silicon dioxide is a nanoparticulate, amorphous form with high surface area, ideal for moisture adsorption in pharmaceuticals. Regular silicon dioxide (e.g., sand) is crystalline and used in bulk industrial applications like glassmaking. Colloidal forms are purer and optimized for biocompatibility in tablets.